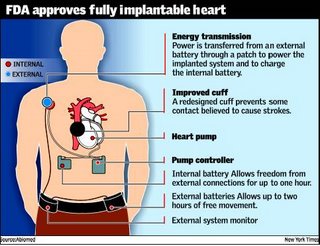
The US food and drug administration gave limited approval on Tuesday to a Massachusetts company to sell the first fully implantable artificial heart, a device that can let patients move about freely for up to two hours at a time.
The approval was given even though the grapefruit-size device was implanted in just 14 patients at four hospitals from 2001 to 2004. All of the patients, who agreed to receive the heart as an experimental device, were men, and all have died. Two died from the implant operation. A third never regained consciousness, and the rest survived an average of five months. The longest survivor lived 512 days, when the mechanical heart failed.
Nevertheless, the agency gave the company, Abiomed Inc. of Danvers, Massachusetts, a humanitarian exemption allowing it to sell up to 4,000 devices a year. The device costs $250,000. Earlier devices were much larger and intended as a bridge to heart transplants. The drug agency has approved a modified version of the heart as a bridge to a transplant. The agency has also approved a partial mechanical heart known as a ventricular assist device for permanent use.
The titanium and plastic Abiomed device can be used just in patients who are near death from the failure of both of the natural heart’s pumping chambers. The device can be implanted only in people 18 and older who are ineligible for a transplant and whose life expectancy would be a month without it.
The diseased heart is removed to make room for the two-pound device. Implanted, a coil transfers power across the skin and recharges the device from the outside. An internal battery and a controller that monitors and controls the heart rate are implanted in the abdomen. The battery allows the recipient to be free from all external connections for up to one hour. Two external batteries allow free movement for up to two hours. But it’s size limits its use to men and women with relatively large chest cavities.
‘Virtually untreatable’ form of TB found
A “virtually untreatable” form of TB has emerged, according to the World Health Organisation (WHO). Extreme drug resistant TB (XDR TB) has been seen worldwide, including in the US, Eastern Europe and Africa. Dr Paul Nunn, from the WHO, said a failure to correctly implement treatment strategies was to blame. TB experts have convened in Johannesburg to discuss how to address the problem. Drug resistance is caused by poor TB control, through taking the wrong types of drugs for the incorrect duration. Multi-drug resistant TB (MDR TB), which describes strains of TB that are resistant to at least two of the main first-line TB drugs, is already a growing concern.
Globally, the WHO estimates there are about 425,000 cases of MDR TB a year, mostly occurring in the former Soviet Union, China and India.Treatment requires the use of second-line drugs, which are more toxic, take longer to work and costly. But now, according to researchers, an even more deadly form of the bacteria has emerged. XDR TB is defined as strains that are not only resistant to the front-line drugs, but also three or more of the six classes of second-line drugs. This, Dr Nunn says makes it virtually untreatable.
A recent survey of 18,000 TB samples by the US-based Centers for Disease Control and the WHO between November 2004 and November 2005 found 20% of them were multi-drug resistant and a further 2% were extreme drug resistant. Further detailed analysis of several countries found the prevalence was even higher.

No comments:
Post a Comment